French PECAN reimbursement applications have started… It’s not a DiGA copy-paste
PECAN – Prise En Charge Anticipée des dispositifs médicaux Numériques (Early reimbursement of digital medical devices)
Like Germany, France has now introduced a dedicated reimbursement fast track for DTx and telemedicine solutions. The process is live since end of March 2023, and by mid June, 14 dossiers were already submitted to the French authorities, despite the fact that pricing rules have not yet been published. While the French have been inspired by the DiGA approach, the new French reimbursement path is not precisely a copy-paste from the neighbours.
By now, many active in the digital health space are familiar with the German DiGA process and ready to find out how the French approach compares. Well, the devil is not always in the detail… Before looking at the precise requirements, evidence dossiers and submission procedures, here are the most fundamental differences in the French and German access paths for digital medical devices:
Route to market
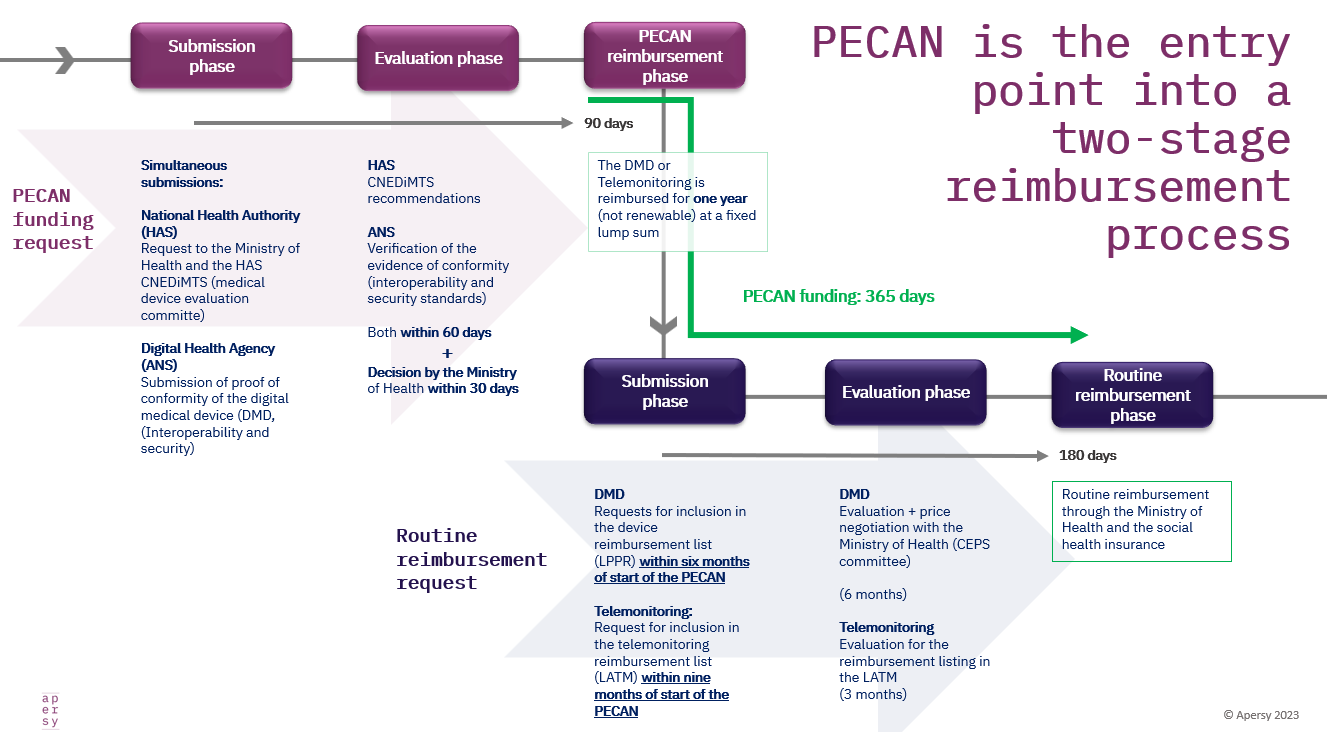
PECAN is not an actual standalone reimbursement path, but an early access tool that needs to be followed by the full routine reimbursement request and process for any medical device. Think of PECAN as interim funding, while DiGA preliminary listing is ‘reimbursement with evidence development’.
Eligibility criteria
While the DiGA reimbursement has a specific scope (most notably device risk class), there are detailed eligibility criteria for the French counterpart, including proof of innovation (in benefit), quality and hence a more substantial baseline evidence level.
Timelines
Even though PECAN interim funding lasts 12 months just like temporary DiGA listing, the full routine reimbursement request with additional evidence must be submitted during the PECAN funding period:
a. Six months after start for DTx
b. Nine months after start for telemedicine solution
Without any option for renewal or delay; and – needless to say, if you don’t submit in time, your PECAN funding also comes to an early end..
Pricing
For DTx, there will be fixed lump sums, not free price setting. However, we are still waiting to hear about the details of how prices are set during the 12 months of PECAN funding…. For telemonitoring, lump sum tariffs have recently been published, including volume tiers and accounting for the benefit claim
But: The two processes do have similarities:
1. They are fast tracks: A PECAN request receives a decision within 90 days
2. PECAN can provide access and funding without full evidence, during the months of remaining evidence collection, HTA review and price negotiations in France
3. Both the PECAN and DiGA process value the structural/organisational advantages that digital devices can offer, in addition to the typical medical benefits.
💡While the DiGA preliminary reimbursement path can be the fall-back option for in-scope patient apps in Germany, PECAN may not be the appropriate route for every digital medical device in France
💡There may be very specific timing situations, indication considerations, price and revenue scenarios where PECAN is possible and/or makes sense.
Any more questions on digital medical devices and healthcare AI?